BAD (タンパク質)
| Pro-apoptotic Bcl-2 protein, BAD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
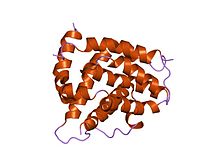 Bad由来ペプチドとBcl-xLの複合体 | |||||||||
| 識別子 | |||||||||
| 略号 | Bcl-2_BAD | ||||||||
| Pfam | PF10514 | ||||||||
| InterPro | IPR018868 | ||||||||
| |||||||||
BAD(BCL2 associated agonist of cell death)[5]はBcl-2ファミリーのアポトーシス促進性のメンバーであり、アポトーシスの開始に関与している。BADはBcl-2ファミリーの中でも、BH3-onlyファミリーのメンバーである[6]。BADは他の多くのBcl-2ファミリーのメンバーと異なり、ミトコンドリア外膜や核膜への標的化を行うC末端の膜貫通ドメインを持たない[7]。活性化後は、抗アポトーシスタンパク質とヘテロ二量体を形成することで、それらによるアポトーシスの停止を防ぐ。
作用機序
[編集]Bax/Bakはミトコンドリア外膜にポアを形成してシトクロムcを細胞質へ放出し、アポトーシスを促進するカスパーゼカスケードを活性化することでアポトーシスを開始すると考えられている。抗アポトーシスタンパク質であるBcl-2やBcl-xLはミトコンドリアのポアを介したシトクロムcの放出を阻害し、またシトクロムcによる細胞質でのカスパーゼカスケードの活性化も阻害する[8]。
非リン酸化状態のBadはBcl-2やBcl-xLとヘテロ二量体を形成し、これらを不活性化してBax/Bakによるアポトーシスの開始を可能にする。BadはAkt/プロテインキナーゼBによるリン酸化(PIP3によって開始される)を受けると、14-3-3タンパク質とヘテロ二量体を形成する。これによってBcl-2はBaxによるアポトーシスを阻害できるようになる[8]。このように、Badのリン酸化は抗アポトーシス作用を持ち、Badの脱リン酸化(Ca2+によって刺激されたカルシニューリンによるものなど)はアポトーシス促進作用を持つ。後者は統合失調症などの神経疾患にも関与している可能性がある[9]。
相互作用
[編集]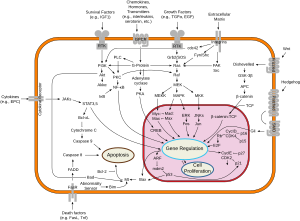
BADは次に挙げる因子と相互作用することが示されている
出典
[編集]- ^ a b c GRCh38: Ensembl release 89: ENSG00000002330 - Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024959 - Ensembl, May 2017
- ^ Human PubMed Reference:
- ^ Mouse PubMed Reference:
- ^ “BAD BCL2 associated agonist of cell death [Homo sapiens (Human)] - Gene - NCBI”. 2021年10月18日閲覧。
- ^ “The proapoptotic BH3-only protein BAD transduces cell death signals independently of its interaction with Bcl-2”. Cell Death Differ. 9 (11): 1240–7. (2002). doi:10.1038/sj.cdd.4401097. PMID 12404123.
- ^ “Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11”. Mol. Endocrinol. 11 (12): 1858–67. (1997). doi:10.1210/me.11.12.1858. PMID 9369453.
- ^ a b Helmreich, E. J. M. (2001). The biochemistry of cell signalling. Oxford: Oxford University Press. pp. 238-243. ISBN 0-19-850820-4. OCLC 45743304
- ^ Foster, T. C.; Sharrow, K. M.; Masse, J. R.; Norris, C. M.; Kumar, A. (2001-06-01). “Calcineurin links Ca2+ dysregulation with brain aging”. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 21 (11): 4066–4073. doi:10.1523/JNEUROSCI.21-11-04066.2001. ISSN 1529-2401. PMC 1201477. PMID 11356894.
- ^ a b c d e f “Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function”. Mol. Cell 17 (3): 393–403. (February 2005). doi:10.1016/j.molcel.2004.12.030. PMID 15694340.
- ^ a b “Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death”. Cell 80 (2): 285–91. (January 1995). doi:10.1016/0092-8674(95)90411-5. PMID 7834748.
- ^ a b c “Underphosphorylated BAD interacts with diverse antiapoptotic Bcl-2 family proteins to regulate apoptosis”. Apoptosis 6 (5): 319–30. (October 2001). doi:10.1023/A:1011319901057. PMID 11483855.
- ^ “Survival function of protein kinase C{iota} as a novel nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-activated bad kinase”. J. Biol. Chem. 280 (16): 16045–52. (April 2005). doi:10.1074/jbc.M413488200. PMID 15705582.
- ^ “BAD partly reverses paclitaxel resistance in human ovarian cancer cells”. Oncogene 17 (19): 2419–27. (November 1998). doi:10.1038/sj.onc.1202180. PMID 9824152.
- ^ “Development of a high-throughput fluorescence polarization assay for Bcl-x(L)”. Anal. Biochem. 307 (1): 70–5. (August 2002). doi:10.1016/S0003-2697(02)00028-3. PMID 12137781.
- ^ a b “The anti-apoptotic molecules Bcl-xL and Bcl-w target protein phosphatase 1alpha to Bad”. Eur. J. Immunol. 32 (7): 1847–55. (July 2002). doi:10.1002/1521-4141(200207)32:7<1847::AID-IMMU1847>3.0.CO;2-7. PMID 12115603.
- ^ “Human homologue of S. pombe Rad9 interacts with BCL-2/BCL-xL and promotes apoptosis”. Nat. Cell Biol. 2 (1): 1–6. (January 2000). doi:10.1038/71316. PMID 10620799.
- ^ “Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies”. Protein Sci. 9 (12): 2528–34. (Dec 2000). doi:10.1110/ps.9.12.2528. PMC 2144516. PMID 11206074.
- ^ “BAD/BCL-[X(L)] heterodimerization leads to bypass of G0/G1 arrest”. Oncogene 20 (33): 4507–18. (July 2001). doi:10.1038/sj.onc.1204584. PMID 11494146.
- ^ “Synergistic anti-apoptotic activity between Bcl-2 and SMN implicated in spinal muscular atrophy”. Nature 390 (6658): 413–7. (November 1997). Bibcode: 1997Natur.390..413I. doi:10.1038/37144. PMID 9389483.
- ^ “PCNA interacts with hHus1/hRad9 in response to DNA damage and replication inhibition”. Oncogene 19 (46): 5291–7. (November 2000). doi:10.1038/sj.onc.1203901. PMID 11077446.
- ^ “Survival activity of Bcl-2 homologs Bcl-w and A1 only partially correlates with their ability to bind pro-apoptotic family members”. Cell Death Differ. 6 (6): 525–32. (June 1999). doi:10.1038/sj.cdd.4400519. PMID 10381646.
- ^ a b “Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11”. Mol. Endocrinol. 11 (12): 1858–67. (November 1997). doi:10.1210/me.11.12.1858. PMID 9369453.
- ^ “The proapoptotic protein Bad binds the amphipathic groove of 14-3-3zeta”. Biochim. Biophys. Acta 1547 (2): 313–9. (June 2001). doi:10.1016/S0167-4838(01)00202-3. PMID 11410287.
関連文献
[編集]- “HIV/SIV escape from immune surveillance: focus on Nef”. Curr. HIV Res. 2 (2): 141–51. (2004). doi:10.2174/1570162043484924. PMID 15078178.
- “p53 and Bad: remote strangers become close friends”. Cell Res. 17 (4): 283–5. (2007). doi:10.1038/cr.2007.19. PMID 17404594.
- “Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death”. Cell 80 (2): 285–91. (1995). doi:10.1016/0092-8674(95)90411-5. PMID 7834748.
- “Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L)”. Cell 87 (4): 619–28. (1996). doi:10.1016/S0092-8674(00)81382-3. PMID 8929531.
- “Bcl-2 targets the protein kinase Raf-1 to mitochondria”. Cell 87 (4): 629–38. (1996). doi:10.1016/S0092-8674(00)81383-5. PMID 8929532.
- “harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L)”. EMBO J. 16 (7): 1686–94. (1997). doi:10.1093/emboj/16.7.1686. PMC 1169772. PMID 9130713.
- “BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity”. J. Biol. Chem. 272 (39): 24101–4. (1997). doi:10.1074/jbc.272.39.24101. PMID 9305851.
- “Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11”. Mol. Endocrinol. 11 (12): 1858–67. (1997). doi:10.1210/me.11.12.1858. PMID 9369453.
- “Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt”. Science 278 (5338): 687–9. (1997). Bibcode: 1997Sci...278..687D. doi:10.1126/science.278.5338.687. PMID 9381178.
- “Dimerization properties of human BAD. Identification of a BH-3 domain and analysis of its binding to mutant BCL-2 and BCL-XL proteins”. J. Biol. Chem. 272 (49): 30866–72. (1997). doi:10.1074/jbc.272.49.30866. PMID 9388232.
- “The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4”. EMBO J. 17 (4): 1029–39. (1998). doi:10.1093/emboj/17.4.1029. PMC 1170452. PMID 9463381.
- “The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136”. Curr. Biol. 8 (13): 779–82. (1998). doi:10.1016/S0960-9822(98)70302-1. PMID 9651683.
- “BAD partly reverses paclitaxel resistance in human ovarian cancer cells”. Oncogene 17 (19): 2419–27. (1998). doi:10.1038/sj.onc.1202180. PMID 9824152.
- “Boo, a novel negative regulator of cell death, interacts with Apaf-1”. EMBO J. 18 (1): 167–78. (1999). doi:10.1093/emboj/18.1.167. PMC 1171112. PMID 9878060.
- “BNIP3alpha: a human homolog of mitochondrial proapoptotic protein BNIP3”. Cancer Res. 59 (3): 533–7. (1999). PMID 9973195.
- “Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD”. Science 284 (5412): 339–43. (1999). Bibcode: 1999Sci...284..339W. doi:10.1126/science.284.5412.339. PMID 10195903.
- “Survival activity of Bcl-2 homologs Bcl-w and A1 only partially correlates with their ability to bind pro-apoptotic family members”. Cell Death Differ. 6 (6): 525–32. (1999). doi:10.1038/sj.cdd.4400519. PMID 10381646.
- “alpha-Synuclein shares physical and functional homology with 14-3-3 proteins”. J. Neurosci. 19 (14): 5782–91. (1999). doi:10.1523/JNEUROSCI.19-14-05782.1999. PMC 6783081. PMID 10407019.
- “Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase”. J. Biol. Chem. 274 (43): 31108–13. (1999). doi:10.1074/jbc.274.43.31108. PMID 10521512.
- “Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms”. Science 286 (5443): 1358–62. (1999). doi:10.1126/science.286.5443.1358. PMID 10558990.
関連項目
[編集]外部リンク
[編集]- bcl-Associated Death Protein - MeSH・アメリカ国立医学図書館・生命科学用語シソーラス
- Human BAD genome location and BAD gene details page in the UCSC Genome Browser.






