利用者:LiterateGiggle/sandbox/esketamine
 |
ここはLiterateGiggleさんの利用者サンドボックスです。編集を試したり下書きを置いておいたりするための場所であり、百科事典の記事ではありません。ただし、公開の場ですので、許諾されていない文章の転載はご遠慮ください。
登録利用者は自分用の利用者サンドボックスを作成できます(サンドボックスを作成する、解説)。 その他のサンドボックス: 共用サンドボックス | モジュールサンドボックス 記事がある程度できあがったら、編集方針を確認して、新規ページを作成しましょう。 |
 | |
 | |
| IUPAC命名法による物質名 | |
|---|---|
| |
| 臨床データ | |
| 販売名 | Spravato, Ketanest, others |
| Drugs.com | monograph |
| MedlinePlus | a619017 |
| ライセンス | EMA:リンク、US Daily Med:リンク |
| 胎児危険度分類 | |
| 法的規制 | |
| データベースID | |
| CAS番号 |
33643-46-8 33643-47-9 |
| ATCコード | N01AX14 (WHO) N06AX27 (WHO) |
| PubChem | CID: 182137 |
| IUPHAR/BPS | 9152 |
| DrugBank |
DB01221 |
| ChemSpider |
158414 |
| UNII |
50LFG02TXD |
| KEGG |
D07283 |
| ChEBI |
CHEBI:60799 |
| ChEMBL |
CHEMBL395091 |
| PDB ligand ID | JC9 (PDBe, RCSB PDB) |
| 別名 | (S)-Ketamine; S(+)-Ketamine; JNJ-54135419 |
| 化学的データ | |
| 化学式 | C13H16ClNO |
| 分子量 | 237.73 g·mol−1 |
| |
エスケタミン( (S)-ケタミン,S(+)-ケタミン等とも表記)は,ケタミンのS(+)-鏡像異性体で[9][10],解離性麻酔薬として全身麻酔に,またうつ病に対する抗うつ薬として用いられる.日本では薬事承認されていないが,承認を受けている国では Spravato や Ketanest といった商品名で発売されている
Esketamine, also known as (S)-ketamine or S(+)-ketamine, is the S(+) enantiomer of ketamine,[9][10] is a dissociative hallucinogen drug used as a general anesthetic and as an antidepressant for treatment of depression. It is sold under the brand names Spravato (for depression), Ketanest (for anesthesia), among others.[7][9][11][12] Esketamine is the active enantiomer of ketamine in terms of NMDA receptor antagonism and is more potent than racemic ketamine.[13]
It is specifically used as a therapy for treatment-resistant depression (TRD) and for major depressive disorder (MDD) with co-occurring suicidal ideation or behavior.[7][14] Its effectiveness for depression is modest and similar to that of other antidepressants.[15][7] Esketamine is not used by infusion into a vein for anesthesia as it is only FDA approved for depression in the form of an intranasal spray (the parent compound Ketamine is most often administered intravenously) and under direct medical supervision as a nasal spray.[7][9]
Adverse effects of esketamine include dissociation, dizziness, sedation, nausea, vomiting, vertigo, numbness, anxiety, lethargy, increased blood pressure, and feelings of drunkenness.[7] Less often, esketamine can cause bladder problems.[7][16] Esketamine acts primarily as a N-methyl-D-aspartate (NMDA) receptor antagonist but also has other actions.[9][10]
In the form of racemic ketamine, esketamine was first synthesized in 1962 and introduced for medical use as an anesthetic in 1970.[17] Enantiopure esketamine was introduced for medical use as an anesthetic in 1997 and as an antidepressant in 2019.[9][7][18] It is used as an anesthetic in the European Union and as an antidepressant in the United States and Canada.[18][19][20] Due to misuse liability as a dissociative hallucinogen, esketamine is a controlled substance.[17][7]
Medical uses
[編集]Anesthesia
[編集]Esketamine is used for similar indications as ketamine.[9] Such uses include induction of anesthesia in high-risk patients such as those with circulatory shock, severe bronchospasm, or as a supplement to regional anesthesia with incomplete nerve blocks.[9]
Depression
[編集]Esketamine is approved under the brand name Spravato in the form of a nasal spray added to a conventional antidepressant as a therapy for treatment-resistant depression (TRD) as well as major depressive disorder (MDD) associated with suicidal ideation or behavior in adults in the United States.[7] In the clinical trials that led to approval of esketamine, TRD was defined as MDD with inadequate response to at least two different conventional antidepressants.[7] The nasal spray formulation of esketamine used for depression delivers two sprays containing a total of 28 mg esketamine and doses of 56 mg (2 devices) to 84 mg (3 devices) are used.[7] The recommended dosage of Spravato is 56 mg on day 1, 56 or 84 mg twice per week during weeks 1 to 4, 56 or 84 mg once per week during weeks 5 to 8, and 56 or 84 mg every 2 weeks or once weekly during week 9 and thereafter.[7] Dosing is individualized to the least frequent dosing necessary to maintain response or remission.[7] Spravato is administered under the supervision of a healthcare provider and patients are monitored for at least 2 hours during each treatment session.[7] Due to concerns about sedation, dissociation, and misuse, esketamine is available for treatment of depression only from certified providers through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called Spravato REMS.[7]
Five clinical studies of esketamine for TRD (TRANSFORM-1, -2, and -3, and SUSTAIN-1 and -2) were submitted to and evaluated by the FDA when approval of esketamine for treatment of TRD was sought by Janssen Pharmaceuticals.[21][22] Of these five studies, three were short-term (4-week) efficacy studies (the TRANSFORM studies).[21][23][22] Two of these three studies (TRANSFORM-1 and -3) did not find a statistically significant antidepressant effect of esketamine relative to placebo.[21][23][15][22] In the one positive short-term efficacy study (TRANSFORM-2), there was a 4.0-point difference between esketamine and placebo on the Montgomery–Åsberg Depression Rating Scale (MADRS) after 4 weeks of treatment (P = 0.020).[21][23][7][22] This scale ranges from 0 to 60 and the average score of the participants at the start of the study was about 37.0 in both the esketamine and placebo groups.[21][23][7] The total change in score after 4 weeks was –19.8 points in the esketamine group and –15.8 points in the placebo group.[21][7] This corresponded to a percentage change in MADRS score from baseline of –53.5% with esketamine and –42.4% with placebo (a difference and reduction of depression score of –11.1% potentially attributable to the pharmacological action of esketamine) in these patient samples.[15][7] Placebo showed 80.0% of the antidepressant effect of esketamine for TRD in this study and hence approximately 20.0% of the antidepressant response was attributable to esketamine.[21][7][24] In the two negative short-term efficacy trials that did not reach statistical significance (TRANSFORM-1 and -3), the differences in MADRS reductions between esketamine and placebo were –3.2 (P = 0.088) and –3.6 (P = 0.059) after 4 weeks of treatment.[22]
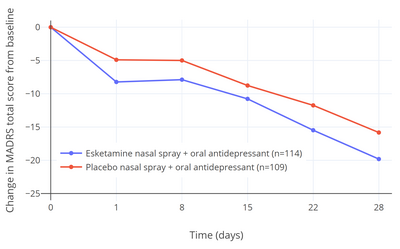
The 4.0-point additional reduction in MADRS score with esketamine over placebo in the single positive efficacy trial corresponds to less than "minimal improvement" and has been criticized as being below the threshold for clinically meaningful change.[21][23] A difference of at least 6.5 points was originally suggested by the trial investigators to be a reasonable threshold for clinical significance.[23][21] In other literature, MADRS reductions have been interpreted as "very much improved" corresponding to 27–28 points, "much improved" to 16–17 points, and "minimally improved" to 7–9 points.[26] It has additionally been argued that the small advantage in scores with esketamine may have been related to an enhanced placebo response in the esketamine group due to functional unblinding caused by the psychoactive effects of esketamine.[21][14][27] In other words, it is argued that the study was not truly a double-blind controlled trial.[21][14] Dissociation was experienced as a side effect by a majority of participants who received esketamine (61–75% with esketamine and 5–12% with placebo; ~7-fold difference) and "severe" dissociation was experienced by 25%.[21][23][7] Deblinding and expectancy confounds are problems with studies of hallucinogens for psychiatric indications in general.[28][29] The FDA normally requires at least two positive short-term efficacy studies for approval of antidepressants, but this requirement was loosened for esketamine and a relapse-prevention trial was allowed to fill the place of the second efficacy trial instead.[21][23] This is the first time that the FDA is known to have made such an exception and the decision has been criticized as lowering regulatory standards.[23] In the relapse-prevention trial (SUSTAIN-2), the rate of depression relapse was significantly lower with esketamine continued than with it discontinued and replaced with placebo in esketamine-treated stable responders and remitters (51% rate reduction in remitters and 70% reduction in responders).[7][23][22]
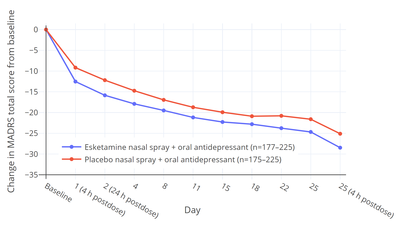
Esketamine was approved for the treatment of MDD with co-occurring suicidal ideation or behavior on the basis of two short-term (4-week) phase 3 trials (ASPIRE-1 and -2) of esketamine nasal spray added to a conventional antidepressant.[7][14][31][30] The primary efficacy measure was reduction in MADRS total score after 24 hours following the first dose of esketamine.[7] In both trials, MADRS scores were significantly reduced with esketamine relative to placebo at 24 hours.[7] The mean MADRS scores at baseline were 39.4 to 41.3 in all groups and the MADRS reductions at 24 hours were –15.9 and –16.0 with esketamine and –12.0 and –12.2 with placebo, resulting in mean differences between esketamine and placebo of –3.8 and –3.9.[7] The secondary efficacy measure in the trials was change in Clinical Global Impression of Suicidal Severity - Revised (CGI-SS-r) 24 hours after the first dose of esketamine.[7] The CGI-SS-r is a single-item scale with scores ranging from 0 to 6.[14] Esketamine was not significantly effective in reducing suicidality relative to placebo on this measure either at 24 hours or after 25 days.[7][30][14] At 24 hours, CGI-SS-r scores were changed by –1.5 with esketamine and –1.3 with placebo, giving a non-significant mean difference between esketamine and placebo of –0.20.[14] Hence, while efficacious in reducing depressive symptoms in people with depression and suicidality, antisuicidal effects of esketamine in such individuals have not been demonstrated.[7][14]
Expectations were initially very high for ketamine and esketamine for treatment of depression based on early small-scale clinical studies, with discovery of the rapid and ostensibly robust antidepressant effects of ketamine described by some authors as "the most important advance in the field of psychiatry in the past half century".[32][33][34] According to a 2018 review, ketamine showed more than double the antidepressant effect size over placebo of conventional antidepressants in the treatment of depression based on the preliminary evidence available at the time (Cohen's d = 1.3–1.7 for ketamine, Cohen's d = 0.8 for midazolam (active placebo), and Cohen's d = 0.53–0.81 for conventional antidepressants).[32] However, the efficacy of ketamine/esketamine for depression declined dramatically as studies became larger and more methodologically rigorous.[15][35] The effectiveness of esketamine for the indication of TRD is described as "modest" and is similar in magnitude to that of other antidepressants for treatment of MDD.[15] The comparative effectiveness of ketamine and esketamine in the treatment of depression has not been adequately characterized.[14] A January 2021 meta-analysis reported that ketamine was similarly effective to esketamine in terms of antidepressant effect size (SMD for depression score of –1.1 vs. –1.2) but more effective than esketamine in terms of response and remission rates (RR = 3.01 vs. RR = 1.38 for response and RR = 3.70 vs. RR = 1.47 for remission).[36][14][37] A September 2021 Cochrane review found that ketamine had an effect size (SMD) for depression at 24 hours of –0.87, with very low certainty, and that esketamine had an effect size (SMD) at 24 hours of –0.31, based on moderate-certainty evidence.[38] However, these meta-analyses have involved largely non-directly-comparative studies with dissimilar research designs and patient populations.[36][14][37] Only a single clinical trial has directly compared ketamine and esketamine for depression as of May 2021.[39][14][40] This study reported similar antidepressant efficacy as well as tolerability and psychotomimetic effects between the two agents.[39][14][40] However, the study was small and underpowered, and more research is still needed to better-characterize the comparative antidepressant effects of ketamine and esketamine.[39][14][40][36][37] Preliminary research suggests that arketamine, the R(−) enantiomer of ketamine, may also have its own independent antidepressant effects and may contribute to the antidepressant efficacy of racemic ketamine, but more research likewise is needed to evaluate this possibility.[41][42]
In February 2019, an outside panel of experts recommended in a 14–2 vote that the FDA approve the nasal spray version of esketamine for TRD, provided that it be given in a clinical setting, with people remaining on site for at least two hours after.[43][44] The reasoning for this requirement is that trial participants temporarily experienced sedation, visual disturbances, trouble speaking, confusion, numbness, and feelings of dizziness during immediately after.[45] The approval of esketamine for TRD by the FDA was controversial due to limited and mixed evidence of efficacy and safety.[44][23][21][24] In January 2020, esketamine was rejected by the National Health Service (NHS) of Great Britain.[46] The NHS questioned the benefits of the medication for depression and claimed that it was too expensive.[46] People who have been already using esketamine were allowed to complete treatment if their doctors considered this necessary.[46]
Spravato debuted to a cost of treatment of US$32,400 per year when it launched in the United States in March 2019.[47] The Institute for Clinical and Economic Review (ICER), which evaluates cost effectiveness of drugs analogously to the National Institute for Health and Care Excellence (NICE) in the United Kingdom, declined to recommend esketamine for depression due to its steep cost and modest efficacy, deeming it not sufficiently cost-effective.[47][48]
Esketamine is the second drug to be approved for TRD by the FDA, following olanzapine/fluoxetine (Symbyax) in 2009.[24][49] Other agents, like the atypical antipsychotics aripiprazole (Abilify) and quetiapine (Seroquel), have been approved for use in the adjunctive therapy of MDD in people with a partial response to treatment.[24] In a meta-analysis conducted internally by the FDA during its evaluation of esketamine for TRD, the FDA reported a standardized mean difference (SMD) of esketamine for TRD of 0.28 using the three phase 3 short-term efficacy trials conducted by Janssen.[24] This was similar to an SMD of 0.26 for olanzapine/fluoxetine for TRD and lower than SMDs of 0.35 for aripiprazole and 0.40 for quetiapine as adjuncts for MDD.[24] These drugs are less expensive than esketamine and may serve as more affordable alternatives to it for depression with similar effectiveness.[24]
Adverse effects
[編集]The most common adverse effects of esketamine for depression (≥5% incidence) include dissociation, dizziness, sedation, nausea, vomiting, vertigo, numbness, anxiety, lethargy, increased blood pressure, and feelings of drunkenness.[7] Long-term use of esketamine has been associated with bladder disease.[7][16]
Pharmacology
[編集]Pharmacodynamics
[編集]Esketamine is approximately twice as potent an anesthetic as racemic ketamine.[50]
In mice, the rapid antidepressant effect of arketamine was greater and lasted longer than that of esketamine.[51] The usefulness of arketamine over esketamine has been supported by other researchers.[52][53][54]
Esketamine inhibits dopamine transporters eight times more than arketamine.[55] This increases dopamine activity in the brain. At doses causing the same intensity of effects, esketamine is generally considered to be more pleasant by patients.[56][57] Patients also generally recover mental function more quickly after being treated with pure esketamine, which may be a result of the fact that it is cleared from their system more quickly.[50][58] This is however in contradiction with arketamine being devoid of psychotomimetic side effects.[59]
Unlike arketamine, esketamine does not bind significantly to sigma receptors. Esketamine increases glucose metabolism in the frontal cortex, while arketamine decreases glucose metabolism in the brain. This difference may be responsible for the fact that esketamine generally has a more dissociative or hallucinogenic effect while arketamine is reportedly more relaxing.[58] However, another study found no difference between racemic ketamine and esketamine on the patient's level of vigilance.[56] Interpretation of this finding is complicated by the fact that racemic ketamine is 50% esketamine.[60]
Pharmacokinetics
[編集]Esketamine is eliminated from the human body more quickly than arketamine (R(–)-ketamine) or racemic ketamine, although arketamine slows the elimination of esketamine.[61]
History
[編集]Esketamine was introduced for medical use as an anesthetic in Germany in 1997, and was subsequently marketed in other countries.[9][19] In addition to its anesthetic effects, the medication showed properties of being a rapid-acting antidepressant, and was subsequently investigated for use as such.[62][63] Esketamine received a breakthrough designation from the FDA for treatment-resistant depression (TRD) in 2013 and major depressive disorder (MDD) with accompanying suicidal ideation in 2016.[63][64] In November 2017, it completed phase III clinical trials for treatment-resistant depression in the United States.[62][63] Johnson & Johnson filed a Food and Drug Administration (FDA) New Drug Application (NDA) for approval on 4 September 2018;[65] the application was endorsed by an FDA advisory panel on 12 February 2019, and on 5 March 2019, the FDA approved esketamine, in conjunction with an oral antidepressant, for the treatment of depression in adults.[18] In August 2020, it was approved by the U.S. Food and Drug Administration (FDA) with the added indication for the short-term treatment of suicidal thoughts.[66]
Since the 1980s, closely associated ketamine has been used as a club drug also known as "Special K" for its trip-inducing side effects.[67][68]
Society and culture
[編集]Names
[編集]Esketamine is the generic name of the drug and its INN and BAN, while esketamine hydrochloride is its BANM.[19] It is also known as S(+)-ketamine, (S)-ketamine, or (–)-ketamine ((-)[+] ketamine) as well as by its developmental code name JNJ-54135419.[19][63]
Esketamine is sold under the brand name Spravato for use as an antidepressant and the brand names Eskesia, Ketanest, Ketanest S, Ketanest-S, Keta-S for use as an anesthetic (veterinary), among others.[19]
Availability
[編集]Esketamine is marketed as an antidepressant in the United States;[18] and as an anesthetic in the European Union.[19]
Legal status
[編集]Esketamine is a Schedule III controlled substance in the United States.[7]
References
[編集]- ^ a b “Spravato”. Therapeutic Goods Administration (TGA) (17 March 2021). 8 September 2021閲覧。
- ^ a b “AusPAR: Esketamine hydrochloride”. Therapeutic Goods Administration (TGA) (24 May 2021). 8 September 2021閲覧。
- ^ “Regulatory Decision Summary - Spravato -”. Health Canada (23 October 2014). 5 June 2022閲覧。
- ^ “Spravato EPAR”. European Medicines Agency (EMA) (16 October 2019). 24 November 2020閲覧。
- ^ “Spravato 28 mg nasal spray, solution - Summary of Product Characteristics (SmPC)”. (emc). 24 November 2020閲覧。
- ^ “Vesierra 25 mg/ml solution for injection/infusion - Summary of Product Characteristics (SmPC)”. (emc) (21 February 2020). 21 April 2021時点のオリジナルよりアーカイブ。24 November 2020閲覧。
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj “Spravato- esketamine hydrochloride solution”. DailyMed (6 August 2020). 26 September 2020閲覧。
- ^ “Updates to the Prescribing Medicines in Pregnancy database”. Therapeutic Goods Administration (TGA) (12 May 2022). 13 May 2022閲覧。
- ^ a b c d e f g h i “[The clinical use of S-(+)-ketamine--a determination of its place]”. Anästhesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 33 (12): 764–70. (December 1998). doi:10.1055/s-2007-994851. PMID 9893910.
- ^ a b c “Ketamine: A tale of two enantiomers”. J Psychopharmacol 35 (2): 109–123. (February 2021). doi:10.1177/0269881120959644. PMC 7859674. PMID 33155503.
- ^ “Text search results for esketamine: Martindale: The Complete Drug Reference”. MedicinesComplete. London, UK: Pharmaceutical Press. 20 August 2017時点のオリジナルよりアーカイブ。20 August 2017閲覧。
- ^ “Ketamine Hydrochloride”. MedicinesComplete. London, UK: Pharmaceutical Press (9 January 2017). 20 August 2017閲覧。[リンク切れ]
- ^ “Ketamine: teaching an old drug new tricks”. Anesthesia and Analgesia 87 (5): 1186–1193. (November 1998). doi:10.1213/00000539-199811000-00039. PMID 9806706.
- ^ a b c d e f g h i j k l m n “Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation”. Am J Psychiatry 178 (5): 383–399. (May 2021). doi:10.1176/appi.ajp.2020.20081251. PMC 9635017. PMID 33726522. "A legitimate criticism, as it relates to interpreting the effect sizes reported with single or repeat-dose ketamine in TRD, is the possibility that nonspecific effects such as functional unblinding (e.g., by patients experiencing dissociation or euphoric responses) and expectancymayinadvertentlyinflate the efficacy of ketamine (51, 52). [...] Given the absence of an adequately designed head-to-head trial, the relative efficacies of intranasal esketamine and intravenous racemic ketamine are not known (65). [...] A recent meta-analysis comparing intranasal and intravenous ketamine formulations was unable to identify a significant difference between formulations as well as routes of delivery in efficacy at 24 hours, 7 days, and 28 days (17). A separate meta-analysis concluded that intravenous ketamine may be superior in efficacy and have lower dropout rates (66). However, it is difficult to draw definitive conclusions from these analyses given the heterogeneity across component studies."
- ^ a b c d e “Consistently Modest Antidepressant Effects in Clinical Trials: the Role of Regulatory Requirements”. Psychopharmacol Bull 51 (3): 79–108. (June 2021). PMC 8374926. PMID 34421147. "Even drugs with novel mechanisms of action such as the esketamine nasal spray show the same effect size and look nearly identical to other antidepressants when evaluated in the regulatory context (42% symptom reduction with placebo, 54% with drug, effect size 0.29). However, it must be taken under consideration that this trial was unique from the others in that it was an adjunctive study of esketamine nasal spray in treatment resistant patients. It is worth noting that two shortterm trials conducted for regulatory approval of esketamine but not included in the label did not reach statistical significance (P = 0.058 and P = 0.088).28 Independent analysis of these esketamine trial data submitted to the FDA show that despite expectations from smallscale preliminary studies, esketamine performs modestly in patients with treatment resistant depression in the context of large, regulatory trials.29 These authors also raised concerns about the potential lack of specificity of drug effects and the risk of side effects demonstrated in these trials. [...] False negatives are well-known risks of small sized studies. However, it is equally important to note that if we do not enroll adequate sample sizes we will continue run the serious risk of getting an inflated false positive resulting in an overestimate of treatment effects that is not replicable (as was the case with many of the earlier regulatory trials, which tended to have small sample sizes).25 This is especially pertinent for early pilot studies of investigational antidepressants (phase I and II trials), which are not always subject to the same regulatory statutes of later stage trials. This phenomenon is illustrated by the dramatic decline of treatment effect sizes seen with esketamine over the course of development (from small pilot studies to large regulatory trials). Although regulatory agencies allow for more lenient methods for exploratory purposes, this method may yield misleading conclusions because these small trials are invariably under-powered. Specifically, these exploratory trials may end up with an erroneously low placebo response and thus a falsely inflated estimate of effect size.46 This possibility is under appreciated by many investigators but should be strongly considered given the persistence of modest effect sizes in regulatory trials of antidepressants."
- ^ a b “Ketamine-induced urological toxicity: potential mechanisms and translation for adults with mood disorders receiving ketamine treatment”. Psychopharmacology (Berl) 238 (4): 917–926. (April 2021). doi:10.1007/s00213-021-05767-1. PMID 33484298.
- ^ a b “Classics in Chemical Neuroscience: Ketamine”. ACS Chem Neurosci 8 (6): 1122–1134. (June 2017). doi:10.1021/acschemneuro.7b00074. PMID 28418641.
- ^ a b c d "FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor's office or clinic". U.S. Food and Drug Administration (FDA) (Press release). 2019年3月6日閲覧。
- ^ a b c d e f “Esketamine”. Drugs.com. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ “The Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force Recommendations for the Use of Racemic Ketamine in Adults with Major Depressive Disorder: Recommandations Du Groupe De Travail Du Réseau Canadien Pour Les Traitements De L'humeur Et De L'anxiété (Canmat) Concernant L'utilisation De La Kétamine Racémique Chez Les Adultes Souffrant De Trouble Dépressif Majeur”. Can J Psychiatry 66 (2): 113–125. (November 2020). doi:10.1177/0706743720970860. PMC 7918868. PMID 33174760.
- ^ a b c d e f g h i j k l m n o “Are we repeating mistakes of the past? A review of the evidence for esketamine”. Br J Psychiatry 219 (5): 614–617. (May 2020). doi:10.1192/bjp.2020.89. PMID 32456714.
- ^ a b c d e f g “Efficacy and Safety of Intranasal Esketamine in Treatment-Resistant Depression in Adults: A Systematic Review”. Cureus 13 (8): e17352. (August 2021). doi:10.7759/cureus.17352. PMC 8381465. PMID 34447651.
- ^ a b c d e f g h i j k l “Esketamine for treatment resistant depression: a trick of smoke and mirrors?”. Epidemiol Psychiatr Sci 29: e79. (December 2019). doi:10.1017/S2045796019000751. PMC 8061126. PMID 31841104.
- ^ a b c d e f g “Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval”. Lancet Psychiatry 6 (12): 977–979. (December 2019). doi:10.1016/S2215-0366(19)30394-3. PMID 31680014.
- ^ “SPRAVATO™ Clinical Studies | Touchstone TMS” (13 January 2020). Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ Paketci, Susan (November 2021). “Interpretation of the Montgomery–Åsberg Depression Rating Scale (MADRS)”. The British Journal of Psychiatry 219 (5): 620–621. doi:10.1192/bjp.2021.162. ISSN 0007-1250. PMID 35048825.
- ^ “The role of dissociation in ketamine's antidepressant effects”. Nat Commun 11 (1): 6431. (December 2020). Bibcode: 2020NatCo..11.6431B. doi:10.1038/s41467-020-20190-4. PMC 7755908. PMID 33353946.
- ^ “Blinding and expectancy confounds in psychedelic randomized controlled trials”. Expert Rev Clin Pharmacol 14 (9): 1133–1152. (September 2021). doi:10.1080/17512433.2021.1933434. PMID 34038314.
- ^ “Who is blind in psychedelic research? Letter to the editor regarding: blinding and expectancy confounds in psychedelic randomized controlled trials”. Expert Rev Clin Pharmacol 14 (10): 1317–1319. (October 2021). doi:10.1080/17512433.2021.1951473. PMID 34227438.
- ^ a b c d “Esketamine Nasal Spray for the Rapid Reduction of Depressive Symptoms in Major Depressive Disorder With Acute Suicidal Ideation or Behavior”. J Clin Psychopharmacol 41 (5): 516–524. (2021). doi:10.1097/JCP.0000000000001465. PMC 8407443. PMID 34412104.
- ^ “Long-Term Efficacy of Intranasal Esketamine in Treatment-Resistant Major Depression: A Systematic Review”. Int J Mol Sci 22 (17): 9338. (August 2021). doi:10.3390/ijms22179338. PMC 8430977. PMID 34502248.
- ^ a b “Antidepressant Efficacy and Tolerability of Ketamine and Esketamine: A Critical Review”. CNS Drugs 32 (5): 411–420. (May 2018). doi:10.1007/s40263-018-0519-3. PMID 29736744. "In brief, these studies (Table 1) have globally assessed responses to a single dose of intravenous ketamine in 166 patients with TDR with multiple treatment failures, including electroconvulsive therapy (ECT). The findings provide evidence of improvement in depressive symptoms within hours, with a response rate > 60% in the first 4.5 and 24 h, and > 40% after 7 days, with a big effect size in comparison with placebo (Cohen's d 1.3–1.7) or active placebo (midazolam, d = 0.8). These figures, though preliminary, contrast with the average effect size of conventional antidepressants (Cohen's d 0.53–0.81 in patients with intense symptoms) [32] and their response latency (about 4–7 weeks) [1]."
- ^ “Esketamine/ketamine for treatment-resistant depression”. Braz J Psychiatry 42 (6): 579–580. (2020). doi:10.1590/1516-4446-2020-0996. PMC 7678896. PMID 32401866. "Some authors have described the discovery of rapid and robust antidepressant effects of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine as the most important advance in the field of psychiatry in the past half century."
- ^ “Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight”. Lancet Psychiatry 4 (5): 419–426. (May 2017). doi:10.1016/S2215-0366(17)30102-5. hdl:10871/30208. PMID 28395988. "Ketamine has been hailed as the most important advance in the treatment of depression of the past 50 years.1"
- ^ “Safety and effectiveness of NMDA receptor antagonists for depression: A multidisciplinary review”. Pharmacotherapy 42 (7): 567–579. (July 2022). doi:10.1002/phar.2707. PMC 9540857. PMID 35665948. "The promising results seen in the small, single-infusion, single-center trials of racemic ketamine were generally not replicated in the larger, multi-site trials of esketamine nasal spray. The esketamine trials were also subject to FDA site inspections, data integrity checks, and other forms of independent scrutiny."
- ^ a b c “Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis”. J Affect Disord 278: 542–555. (January 2021). doi:10.1016/j.jad.2020.09.071. PMC 7704936. PMID 33022440.
- ^ a b c “Comments to Drs. Bahji, Vazquez, and Zarate”. J Affect Disord 283: 262–264. (March 2021). doi:10.1016/j.jad.2021.01.046. PMID 33571795.
- ^ “Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder”. Cochrane Database Syst Rev 9 (11): CD011612. (September 2021). doi:10.1002/14651858.CD011612.pub3. PMC 8434915. PMID 34510411.
- ^ a b c “Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status”. CNS Drugs 35 (5): 527–543. (May 2021). doi:10.1007/s40263-021-00816-x. PMC 8201267. PMID 33904154. "To date, only one study has examined the differences between esketamine (0.25 mg/kg) and (R,S)-ketamine (0.5 mg/kg); though underpowered, it found no differences in efficacy, tolerability, or psychotomimetic profile between the two agents [67]. A recent meta-analysis suggests the need to compare these two agents head-to-head [68]."
- ^ a b c “Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: A randomized, double-blind, non-inferiority study”. J Affect Disord 264: 527–534. (March 2020). doi:10.1016/j.jad.2019.11.086. PMID 31786030.
- ^ “Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine”. Biochem Pharmacol 177: 113935. (July 2020). doi:10.1016/j.bcp.2020.113935. PMID 32224141.
- ^ “Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor”. Mol Psychiatry 27 (1): 559–573. (May 2021). doi:10.1038/s41380-021-01121-1. PMC 8960399. PMID 33963284.
- ^ “First Big Depression Advance Since Prozac Nears FDA Approval.”. Bloomberg News. (12 February 2019) 12 February 2019閲覧。
- ^ a b “Why a ketamine-like drug is being used to treat depression”. Vox (6 March 2019). 27 November 2021閲覧。
- ^ Psychopharmacologic Drugs Advisory Committee (PDAC) and Drug Safety and Risk Management (DSaRM) Advisory Committee (12 February 2019). “FDA Briefing Document”. Food and Drug Administration. 12 February 2019閲覧。 “Meeting, February 12, 2019. Agenda Topic: The committees will discuss the efficacy, safety, and risk-benefit profile of New Drug Application (NDA) 211243, esketamine 28 mg single-use nasal spray device, submitted by Janssen Pharmaceutica, for the treatment of treatment-resistant depression.”
- ^ a b c “Anti-depressant spray not recommended on NHS”. BBC News (28 January 2020). Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ a b “J&J scores Spravato trial win in high-risk depression. Will doctors and payers buy in?”. FiercePharma (10 September 2019). 27 November 2021閲覧。 “Pricing, though, may still be an issue. In early May, the Institute for Clinical and Economic Review (ICER) declined to recommend Spravato for use at its steep list price of $32,400 per year. The U.S. cost watchdog said J&J would need to cut the sticker price between 25% and 52% to be considered cost-effective.”
- ^ “1 Recommendations | Esketamine nasal spray for treatment-resistant depression | Guidance | NICE”. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- ^ “Intranasal esketamine: From origins to future implications in treatment-resistant depression”. J Psychiatr Res 137: 29–35. (May 2021). doi:10.1016/j.jpsychires.2021.02.020. PMID 33647726.
- ^ a b “[The clinical use of S-(+)-ketamine--a determination of its place]” (ドイツ語). Anästhesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 33 (12): 764–70. (December 1998). doi:10.1055/s-2007-994851. PMID 9893910.
- ^ “R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine”. Pharmacology, Biochemistry, and Behavior 116: 137–41. (January 2014). doi:10.1016/j.pbb.2013.11.033. PMID 24316345.
- ^ “Ketamine enantiomers in the rapid and sustained antidepressant effects”. Therapeutic Advances in Psychopharmacology 6 (3): 185–92. (June 2016). doi:10.1177/2045125316631267. PMC 4910398. PMID 27354907.
- ^ “Ketamine's antidepressant action: beyond NMDA receptor inhibition”. Expert Opinion on Therapeutic Targets 20 (11): 1389–1392. (November 2016). doi:10.1080/14728222.2016.1238899. PMID 27646666.
- ^ “Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression”. Psychopharmacology 233 (19–20): 3647–57. (October 2016). doi:10.1007/s00213-016-4399-2. PMC 5021744. PMID 27488193.
- ^ “Ketamine stereoselectively inhibits rat dopamine transporter”. Neuroscience Letters 274 (2): 131–4. (October 1999). doi:10.1016/s0304-3940(99)00688-6. PMID 10553955.
- ^ a b “[Ketamine racemate or S-(+)-ketamine and midazolam. The effect on vigilance, efficacy and subjective findings]” (ドイツ語). Der Anaesthesist 41 (10): 610–8. (October 1992). PMID 1443509.
- ^ “[Psychometric changes as well as analgesic action and cardiovascular adverse effects of ketamine racemate versus s-(+)-ketamine in subanesthetic doses]” (ドイツ語). Der Anaesthesist 43 (Suppl 2): S68-75. (November 1994). PMID 7840417.
- ^ a b “Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET)”. European Neuropsychopharmacology 7 (1): 25–38. (February 1997). doi:10.1016/s0924-977x(96)00042-9. PMID 9088882.
- ^ “R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects”. Translational Psychiatry 5 (9): e632. (September 2015). doi:10.1038/tp.2015.136. PMC 5068814. PMID 26327690.
- ^ “The Nuances of Ketamine's Neurochemistry” (英語). Psychedelic Science Review (15 February 2021). 16 February 2021閲覧。
- ^ “Stereoselective pharmacokinetics of ketamine: R(–)-ketamine inhibits the elimination of S(+)-ketamine”. Clinical Pharmacology and Therapeutics 70 (5): 431–8. (November 2001). doi:10.1067/mcp.2001.119722. PMID 11719729.
- ^ a b “Beyond serotonin: newer antidepressants in the future”. Expert Review of Neurotherapeutics 17 (8): 777–790. (August 2017). doi:10.1080/14737175.2017.1341310. PMID 28598698.
- ^ a b c d “Esketamine - Johnson & Johnson - AdisInsight”. 7 November 2017閲覧。
- ^ “Ketamine and Beyond: Investigations into the Potential of Glutamatergic Agents to Treat Depression”. Drugs 77 (4): 381–401. (March 2017). doi:10.1007/s40265-017-0702-8. PMC 5342919. PMID 28194724.
- ^ “Janssen Submits Esketamine Nasal Spray New Drug Application to U.S. FDA for Treatment-Resistant Depression”. Janssen Pharmaceuticals, Inc.. 14 August 2020時点のオリジナルよりアーカイブ。12 February 2019閲覧。
- ^ “FDA Approves A Nasal Spray To Treat Patients Who Are Suicidal”. NPR.org. (4 August 2020) 27 September 2020閲覧。
- ^ “A Paradigm Shift for Depression Treatment”. Discover (Kalmbach Media). (January 2020).
- ^ “The FDA Approved a Ketamine-Like Nasal Spray for Hard-to-Treat Depression”. Vice. (7 March 2019) 11 February 2020閲覧。.
External links
[編集]- “Esketamine”. Drug Information Portal. U.S. National Library of Medicine. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- “Esketamine hydrochloride”. Drug Information Portal. U.S. National Library of Medicine. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
