利用者:Carrot031/sandbox
 |
ここはCarrot031さんの利用者サンドボックスです。編集を試したり下書きを置いておいたりするための場所であり、百科事典の記事ではありません。ただし、公開の場ですので、許諾されていない文章の転載はご遠慮ください。
登録利用者は自分用の利用者サンドボックスを作成できます(サンドボックスを作成する、解説)。 その他のサンドボックス: 共用サンドボックス | モジュールサンドボックス 記事がある程度できあがったら、編集方針を確認して、新規ページを作成しましょう。 |

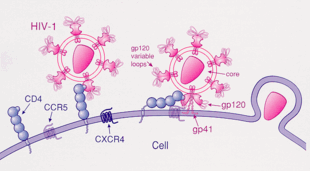
C-C ケモシン受容体5(CCR5 や CD195としても知られる) は白血球の表面のタンパク質であり、免疫系に関わっている。 as it acts as a receptor for ケモカインs. This is the process by which T cells are attracted to specific tissue and organ targets. Many forms of HIV, the virus that causes AIDS, initially use CCR5 to enter and infect host cells. A few individuals carry a mutation known as CCR5-Δ32 in the CCR5 gene, protecting them against these strains of HIV.
In humans, the CCR5 gene that encodes the CCR5 protein is located on the short (p) arm at position 21 on chromosome 3. Certain populations have inherited the Delta 32 mutation resulting in the genetic deletion of a portion of the CCR5 gene. Homozygous carriers of this mutation are resistant to M-tropic strains of HIV-1 infection.[1]
Function
[編集]The CCR5 protein belongs to the beta chemokine receptors family of integral membrane proteins.[2][3] It is a G protein-coupled receptor[2] which functions as a chemokine receptor in the CC chemokine group.
The natural chemokine ligands that bind to this receptor are RANTES (a chemotactic cytokine protein also known as CCL5)[4][5][6] and macrophage inflammatory protein (MIP) 1α and 1β (also known as CCL3 and CCL4). It also interacts with CCL3L1.[5][7]
CCR5 is predominantly expressed on T cells, macrophages, dendritic cells and microglia. It is likely that CCR5 plays a role in inflammatory responses to infection, though its exact role in normal immune function is unclear.
HIV
[編集]HIV most commonly uses CCR5 and/or CXCR4 as a co-receptor to enter its target cells. Several chemokine receptors can function as viral coreceptors, but CCR5 is likely the most physiologically important coreceptor during natural infection. The normal ligands for this receptor, RANTES, MIP-1β, and MIP-1α, are able to suppress HIV-1 infection in vitro. In individuals infected with HIV, CCR5-using viruses are the predominant species isolated during the early stages of viral infection,[8] suggesting that these viruses may have a selective advantage during transmission or the acute phase of disease. Moreover, at least half of all infected individuals harbor only CCR5-using viruses throughout the course of infection.
A number of new experimental HIV drugs, called CCR5 receptor antagonists, have been designed to interfere with the interaction between CCR5 and HIV, including PRO140 (Progenics), Vicriviroc (Schering Plough), Aplaviroc (GW-873140) (GlaxoSmithKline) and Maraviroc (UK-427857) (Pfizer). A problem of this approach is that, while CCR5 is the major co-receptor by which HIV infects cells, it is not the only such co-receptor. It is possible that under selective pressure HIV will evolve to use another co-receptor. However, examination of viral resistance to AD101, molecular antagonist of CCR5, indicated that resistant viruses did not switch to another coreceptor (CXCR4) but persisted in using CCR5, either through binding to alternative domains of CCR5, or by binding to the receptor at a higher affinity.
CCR5-Δ32
[編集]CCR5-Δ32 (or CCR5-D32 or CCR5 delta 32) is an allele of CCR5.[9][10]
CCR5-Δ32 is a deletion mutation of a gene that has a specific impact on the function of T cells.[11] At least one copy of CCR5-Δ32 is found in about 4–16% of people of European descent. It has been speculated that this allele was favored by natural selection during the Black Death for Northern Europeans, but further research has revealed that the gene did not protect against the Black Death.[12] The current hypothesis is of protection vs smallpox throughout Europe,[12] especially in the major trade cities and in isolated islands and archipelagos, such as Iceland and the Azores.[13]
In the ancient world in areas such as Corinth in Ancient Greece, prostitution may have led to infection, since a virus similar to HIV existed which had flu-like symptoms and later continued to weaken the immune system of those infected. It was at the time not known how it was spread but the Plague of Athens and many later diseases in the Balkans may have also influenced the genetic mutations. [14] This coalescence date is contradicted by surported evidence of CCR5-Δ32 in Bronze Age samples, at levels comparable to the modern European population.[15] Smallpox may be another candidate for the high level of the mutation in the European population.[9]
The allele has a negative effect upon T cell function, but appears to protect against smallpox and HIV. Yersinia pestis (the bubonic plague bacterium) was demonstrated in the laboratory not to associate with CCR5. Individuals with the Δ32 allele of CCR5 are healthy, suggesting that CCR5 is largely dispensable. However, CCR5 apparently plays a role in mediating resistance to West Nile virus infection in humans, as CCR5-Δ32 individuals have shown to be disproportionately at higher risk of West Nile virus in studies,[16] indicating that not all of the functions of CCR5 may be compensated by other receptors.
While CCR5 has multiple variants in its coding region, the deletion of a 32-bp segment results in a nonfunctional receptor, thus preventing HIV R5 entry; two copies of this allele provide strong protection against HIV infection.[17] This allele is found in 5–14% of Europeans but is rare in Africans and Asians.[18] CCR5-Δ32 decreases the number of CCR5 proteins on the outside of the CD4 cell, which can have a large effect on the HIV disease progression rates. Multiple studies of HIV-infected persons have shown that presence of one copy of this allele delays progression to the condition of AIDS by about two years. It is possible that a person with the CCR5-Δ32 receptor allele will not be infected with HIV R5 strains. Several commercial testing companies offer tests for CCR5-Δ32.[19]
A genetic approach involving intrabodies that block CCR5 expression has been proposed as a treatment for HIV-1 infected individuals.[20] When T-cells modified so they no longer express CCR5 were mixed with unmodified T-cells expressing CCR5 and then challenged by infection with HIV-1, the modified T-cells that do not express CCR5 eventually take over the culture, as HIV-1 kills the non-modified T-cells. This same method might be used in vivo to establish a virus resistant cell pool in infected individuals.[20]
This hypothesis was tested in an AIDS patient who had also developed myeloid leukemia, and was treated with chemotherapy to suppress the cancer. A bone marrow transplant containing stem cells from a matched donor was then used to restore the immune system. However, the transplant was performed from a donor with 2 copies of CCR5-Δ32 mutation gene. After 600 days, the patient was healthy and had undetectable levels of HIV in the blood and in examined brain and rectal tissues.[21][22] Before the transplant, low levels of HIV X4, which does not use the CCR5 receptor, were also detected. Following the transplant, however, this type of HIV was not detected either, further baffling doctors.[22] However, this is consistent with the observation that cells expressing the CCR5-Δ32 variant protein lack both the CCR5 and CXCR4 receptors on their surfaces, thereby conferring resistance to a broad range of HIV variants including HIV X4.[23] After three years, the patient has maintained the resistance to HIV and has been pronounced cured of the HIV infection.[24]
Enrollment of HIV-positive patients in a clinical trial was started in 2009 in which the patients' cells were genetically modified with a zinc finger nuclease to carry the CCR5-Δ32 trait and then reintroduced into the body as a potential HIV treatment.[25][26]
See also
[編集]References
[編集]- ^ Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth RJ, Collman RG, Doms RW, Vassart G, Parmentier M (August 1996). “Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene”. Nature 382 (6593): 722–5. doi:10.1038/382722a0. PMID 8751444.
- ^ a b Genetics Home Reference
- ^ Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M (March 1996). “Molecular cloning and functional expression of a new human CC-chemokine receptor gene”. Biochemistry 35 (11): 3362–7. doi:10.1021/bi952950g. PMID 8639485.
- ^ Slimani H, Charnaux N, Mbemba E, Saffar L, Vassy R, Vita C, Gattegno L (October 2003). “Interaction of RANTES with syndecan-1 and syndecan-4 expressed by human primary macrophages”. Biochim. Biophys. Acta 1617 (1–2): 80–8. doi:10.1016/j.bbamem.2003.09.006. PMID 14637022.
- ^ a b Struyf S, Menten P, Lenaerts JP, Put W, D'Haese A, De Clercq E, Schols D, Proost P, Van Damme J (July 2001). “Diverging binding capacities of natural LD78beta isoforms of macrophage inflammatory protein-1alpha to the CC chemokine receptors 1, 3 and 5 affect their anti-HIV-1 activity and chemotactic potencies for neutrophils and eosinophils”. Eur. J. Immunol. 31 (7): 2170–8. doi:10.1002/1521-4141(200107)31:7<2170::AID-IMMU2170>3.0.CO;2-D. PMID 11449371.
- ^ Proudfoot AE, Fritchley S, Borlat F, Shaw JP, Vilbois F, Zwahlen C, Trkola A, Marchant D, Clapham PR, Wells TN (April 2001). “The BBXB motif of RANTES is the principal site for heparin binding and controls receptor selectivity”. J. Biol. Chem. 276 (14): 10620–6. doi:10.1074/jbc.M010867200. PMID 11116158.
- ^ Miyakawa T, Obaru K, Maeda K, Harada S, Mitsuya H (February 2002). “Identification of amino acid residues critical for LD78beta, a variant of human macrophage inflammatory protein-1alpha, binding to CCR5 and inhibition of R5 human immunodeficiency virus type 1 replication”. J. Biol. Chem. 277 (7): 4649–55. doi:10.1074/jbc.M109198200. PMID 11734558.
- ^ Anderson J, Akkina R (2007). “Complete Knockdown of CCR5 by lentiviral vector-expressed siRNAs and protection of transgenic macrophages against HIV-1 infection”. Gene Therapy 14 (14): 1287–1297. doi:10.1038/sj.gt.3302958. PMID 17597795.
- ^ a b Galvani AP, Slatkin M (December 2003). “Evaluating plague and smallpox as historical selective pressures for the CCR5-Delta 32 HIV-resistance allele”. Proc. Natl. Acad. Sci. U.S.A. 100 (25): 15276–9. doi:10.1073/pnas.2435085100. PMC 299980. PMID 14645720.
- ^ Stephens JC, Reich DE, Goldstein DB, Shin HD, Smith MW, Carrington M, Winkler C, Huttley GA, Allikmets R, Schriml L, Gerrard B, Malasky M, Ramos MD, Morlot S, Tzetis M, Oddoux C, di Giovine FS, Nasioulas G, Chandler D, Aseev M, Hanson M, Kalaydjieva L, Glavac D, Gasparini P, Kanavakis E, Claustres M, Kambouris M, Ostrer H, Duff G, Baranov V, Sibul H, Metspalu A, Goldman D, Martin N, Duffy D, Schmidtke J, Estivill X, O'Brien SJ, Dean M (June 1998). “Dating the origin of the CCR5-Delta32 AIDS-resistance allele by the coalescence of haplotypes”. Am. J. Hum. Genet. 62 (6): 1507–15. doi:10.1086/301867. PMC 1377146. PMID 9585595.
- ^ Buseyne F, Janvier G, Teglas JP, Ivanoff S, Burgard M, Bui E, Mayaux MJ, Blanche S, Rouzioux C, Rivière Y (October 1998). “Impact of heterozygosity for the chemokine receptor CCR5 32-bp-deleted allele on plasma virus load and CD4 T lymphocytes in perinatally human immunodeficiency virus-infected children at 8 years of age”. J. Infect. Dis. 178 (4): 1019–23. doi:10.1086/515660. PMID 9806029.
- ^ a b Faure E, Royer-Carenzi M (December 2008). “Is the European spatial distribution of the HIV-1-resistant CCR5-Delta32 allele formed by a breakdown of the pathocenosis due to the historical Roman expansion?”. Infect. Genet. Evol. 8 (6): 864–74. doi:10.1016/j.meegid.2008.08.007. PMID 18790087.
- ^ Freitas T, Brehm A, Fernandes AT (December 2006). “Frequency of the CCR5-delta32 mutation in the Atlantic island populations of Madeira, the Azores, Cabo Verde, and São Tomé e Príncipe”. Hum. Biol. 78 (6): 697–703. doi:10.1353/hub.2007.0011. PMID 17564248.
- ^ Mirko D. Grmek (March 1991). Diseases in the Ancient Greek World
- ^ Hedrick PW, Verrelli BC (June 2006). “"Ground truth" for selection on CCR5-Delta32”. Trends Genet. 22 (6): 293–6. doi:10.1016/j.tig.2006.04.007. PMID 16678299.
- ^ Glass WG, McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, Pape J, Cheshier RC, Murphy PM (January 2006). “CCR5 deficiency increases risk of symptomatic West Nile virus infection”. J. Exp. Med. 203 (1): 35–40. doi:10.1084/jem.20051970. PMC 2118086. PMID 16418398.
- ^ Duncan SR, Scott S, Duncan CJ (March 2005). “Reappraisal of the historical selective pressures for the CCR5-Delta32 mutation”. J. Med. Genet. 42 (3): 205–8. doi:10.1136/jmg.2004.025346. PMC 1736018. PMID 15744032. 非専門家向けの内容要旨 – PhysOrg.com.
- ^ Sabeti PC, Walsh E, Schaffner SF, Varilly P, Fry B, Hutcheson HB, Cullen M, Mikkelsen TS, Roy J, Patterson N, Cooper R, Reich D, Altshuler D, O'Brien S, Lander ES (November 2005). “The case for selection at CCR5-Delta32”. PLoS Biol. 3 (11): e378. doi:10.1371/journal.pbio.0030378. PMC 1275522. PMID 16248677.
- ^ “Delta32 Genetic Testing”. Briefing paper for AFAO members. Australian Federation of AIDS Organisations (2007年6月1日). 2011年1月22日閲覧。
- ^ a b Steinberger P, Andris-Widhopf J, Bühler B, Torbett BE, Barbas CF (January 2000). “Functional deletion of the CCR5 receptor by intracellular immunization produces cells that are refractory to CCR5-dependent HIV-1 infection and cell fusion”. Proc. Natl. Acad. Sci. U.S.A. 97 (2): 805–10. doi:10.1073/pnas.97.2.805. PMC 15412. PMID 10639161.
- ^ Schoofs M (2008年11月7日). “A Doctor, a Mutation and a Potential Cure for AIDS”. The Wall Street Journal 2010年12月15日閲覧。
- ^ a b Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, Schneider T, Hofmann J, Kücherer C, Blau O, Blau IW, Hofmann WK, Thiel E (February 2009). “Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation”. N. Engl. J. Med. 360 (7): 692–8. doi:10.1056/NEJMoa0802905. PMID 19213682. 非専門家向けの内容要旨 – CNN.com.
- ^ Agrawal L, Lu X, Qingwen J, VanHorn-Ali Z, Nicolescu IV, McDermott DH, Murphy PM, Alkhatib G (March 2004). “Role for CCR5Delta32 protein in resistance to R5, R5X4, and X4 human immunodeficiency virus type 1 in primary CD4+ cells”. J. Virol. 78 (5): 2277–87. doi:10.1128/JVI.78.5.2277-2287.2004. PMC 369216. PMID 14963124.
- ^ Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T (December 2010). “Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation”. Blood 117 (10): 2791–2799. doi:10.1182/blood-2010-09-309591. PMID 21148083. 非専門家向けの内容要旨 – New Scientist.
- ^ “Autologous T-Cells Genetically Modified at the CCR5 Gene by Zinc Finger Nucleases SB-728 for HIV (Zinc-Finger)”. U.S. National Institutes of Health (2009年12月9日). 2009年12月30日閲覧。
- ^ Wade, Nicholas (2009年12月28日). “Zinc Fingers Could Be Key to Reviving Gene Therapy”. The New York Times 2009年12月30日閲覧。
Further reading
[編集]- Wilkinson D (1997). “Cofactors provide the entry keys. HIV-1”. Curr. Biol. 6 (9): 1051–3. doi:10.1016/S0960-9822(02)70661-1. PMID 8805353.
- Broder CC, Dimitrov DS (1997). “HIV and the 7-transmembrane domain receptors”. Pathobiology 64 (4): 171–9. doi:10.1159/000164032. PMID 9031325.
- Choe H, Martin KA, Farzan M, Sodroski J, Gerard NP, Gerard C (1998). “Structural interactions between chemokine receptors, gp120 Env and CD4”. Semin. Immunol. 10 (3): 249–57. doi:10.1006/smim.1998.0127. PMID 9653051.
- Sheppard HW, Celum C, Michael NL, O'Brien S, Dean M, Carrington M, Dondero D, Buchbinder SP (2002). “HIV-1 infection in individuals with the CCR5-Delta32/Delta32 genotype: acquisition of syncytium-inducing virus at seroconversion”. J. Acquir. Immune Defic. Syndr. 29 (3): 307–13. PMID 11873082.
- Freedman BD, Liu QH, Del Corno M, Collman RG (2004). “HIV-1 gp120 chemokine receptor-mediated signaling in human macrophages”. Immunol. Res. 27 (2–3): 261–76. doi:10.1385/IR:27:2-3:261. PMID 12857973.
- Esté JA (2004). “Virus entry as a target for anti-HIV intervention”. Curr. Med. Chem. 10 (17): 1617–32. doi:10.2174/0929867033457098. PMID 12871111.
- Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, Blumenthal R (2003). “The HIV Env-mediated fusion reaction”. Biochim. Biophys. Acta 1614 (1): 36–50. doi:10.1016/S0005-2736(03)00161-5. PMID 12873764.
- Zaitseva M, Peden K, Golding H (2003). “HIV coreceptors: role of structure, posttranslational modifications, and internalization in viral-cell fusion and as targets for entry inhibitors”. Biochim. Biophys. Acta 1614 (1): 51–61. doi:10.1016/S0005-2736(03)00162-7. PMID 12873765.
- Lee C, Liu QH, Tomkowicz B, Yi Y, Freedman BD, Collman RG (2004). “Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways”. J. Leukoc. Biol. 74 (5): 676–82. doi:10.1189/jlb.0503206. PMID 12960231.
- Yi Y, Lee C, Liu QH, Freedman BD, Collman RG (2004). “Chemokine receptor utilization and macrophage signaling by human immunodeficiency virus type 1 gp120: Implications for neuropathogenesis”. J. Neurovirol. 10 Suppl 1: 91–6. PMID 14982745.
- Seibert C, Sakmar TP (2004). “Small-molecule antagonists of CCR5 and CXCR4: a promising new class of anti-HIV-1 drugs”. Curr. Pharm. Des. 10 (17): 2041–62. doi:10.2174/1381612043384312. PMID 15279544.
- Cutler CW, Jotwani R (2006). “Oral mucosal expression of HIV-1 receptors, co-receptors, and alpha-defensins: tableau of resistance or susceptibility to HIV infection?”. Adv. Dent. Res. 19 (1): 49–51. doi:10.1177/154407370601900110. PMID 16672549.
- Ajuebor MN, Carey JA, Swain MG (2006). “CCR5 in T cell-mediated liver diseases: what's going on?”. J. Immunol. 177 (4): 2039–45. PMID 16887960.
- Lipp M, Müller G (2006). “Shaping up adaptive immunity: the impact of CCR7 and CXCR5 on lymphocyte trafficking”. Verhandlungen der Deutschen Gesellschaft für Pathologie 87: 90–101. PMID 16888899.
- Balistreri CR, Caruso C, Grimaldi MP, Listì F, Vasto S, Orlando V, Campagna AM, Lio D, Candore G (2007). “CCR5 receptor: biologic and genetic implications in age-related diseases”. Ann. N. Y. Acad. Sci. 1100: 162–72. doi:10.1196/annals.1395.014. PMID 17460174.
- Madsen HO, Poulsen K, Dahl O, Clark BF, Hjorth JP (1990). “Retropseudogenes constitute the major part of the human elongation factor 1 alpha gene family”. Nucleic Acids Res. 18 (6): 1513–6. doi:10.1093/nar/18.6.1513. PMC 330519. PMID 2183196.
- Uetsuki T, Naito A, Nagata S, Kaziro Y (1989). “Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor-1 alpha”. J. Biol. Chem. 264 (10): 5791–8. PMID 2564392.
- Whiteheart SW, Shenbagamurthi P, Chen L, Cotter RJ, Hart GW (1989). “Murine elongation factor 1 alpha (EF-1 alpha) is posttranslationally modified by novel amide-linked ethanolamine-phosphoglycerol moieties. Addition of ethanolamine-phosphoglycerol to specific glutamic acid residues on EF-1 alpha”. J. Biol. Chem. 264 (24): 14334–41. PMID 2569467.
- Ann DK, Wu MM, Huang T, Carlson DM, Wu R (1988). “Retinol-regulated gene expression in human tracheobronchial epithelial cells. Enhanced expression of elongation factor EF-1 alpha”. J. Biol. Chem. 263 (8): 3546–9. PMID 3346208.
- Brands JH, Maassen JA, van Hemert FJ, Amons R, Möller W (1986). “The primary structure of the alpha subunit of human elongation factor 1. Structural aspects of guanine-nucleotide-binding sites”. Eur. J. Biochem. 155 (1): 167–71. doi:10.1111/j.1432-1033.1986.tb09472.x. PMID 3512269.
External links
[編集]- Video and text from a PBS documentary about the discovery of CCR5
- “Chemokine Receptors: CCR5”. IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology. Template:Cite webの呼び出しエラー:引数 accessdate は必須です。
- HuGENavigator literature on HIV Infections and CCR5 from CDC - (note, authors may not be CDC employees, and there is no public domain notice on the page, so this cannot be assumed to be public domain)
- Schering-Plough Initiates Phase III Studies with CCR5-Vicriviroc in Treatment- Experienced HIV Patients.
- HIVcoPred A server for prediction of HIV coreceptor usage (CCR5). PLoS ONE 8(4): e61437
Template:AIDS Template:Chemokine receptors Template:Clusters of differentiation
